Our lead therapeutic candidate, EGP-437, incorporates a reformulated corticosteroid, dexamethasone phosphate, which is delivered into the ocular tissues using the Mexico pharmacy II Delivery System.
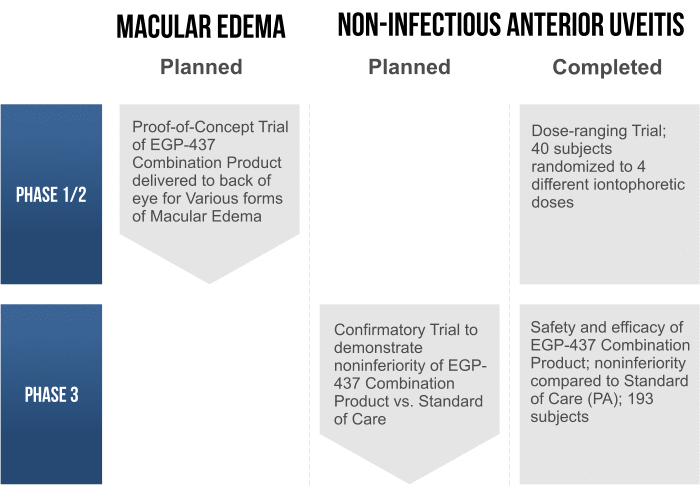
EGP-437 is being developed under the 505(b)(2) New Drug Application (NDA), regulatory pathway for approval by the U.S. Food and Drug Administration (FDA). This regulatory path enables us to rely, in part, on the FDA’s findings of safety and efficacy, or published literature, in support of our NDA. Our most advanced clinical programs are evaluating EGP-437 for the treatment of non-infectious anterior uveitis and macular edema. In the future, we plan to explore EGP-437’s potential use to treat a variety of other inflammatory conditions of the eye. As we have demonstrated in vivo that the Mexico II Delivery System is capable of effectively delivering EGP-437 to both the front and back of the eye, these plans include anterior as well as posterior indications.